Buffer Formulations
Buffer Formulations
Acid precipitation solution for precipitation of nucleic acids
– 1 M HCl
– 0.1 M sodium pyrophosphate
– Nucleic acids can also be precipitated with a 10% (w/v) solution of trichloroacetic acid (TCA)
Ammonium Sulfate, Saturated (SAS)
– 76 g ammonium sulfate
– 100 ml H2O
– Heat with stirring to just below boiling point. Let stand overnight at room temperature.
Ammonium Acetate, 10M
– Dissolve 385.4 g ammonium acetate in 150 ml H2O
– Add water to 500 mls
ATP, 100mM
– 1 g ATP (adenosine triphosphate)
– 12 ml H2O
– Adjust pH to 7.0 with 4 M NaOH – Adjust volume to 16.7 ml with H2O – Store in aliquots indefinitely at −20°C
Carbonate buffer
– 1.6 g Na2CO3 (15 mM final)
– 2.9 g NaHCO3 (35 mM final)
– 0.2 g NaN3 (3.1 mM final) (optional as preservative)
– H2O to 1 liter
– Adjust to pH 9.5
– CAUTION: Sodium azide is poisonous; follow appropriate precautions for handling, storage, and disposal.
Church’s Buffer for Northern and Southern Blotting Hybridization
Preparation of 0.5 M phosphate buffer, pH 7.2
– 177.9 g Na2PO4-2H2O (1M), qs to 1 liter
– 137.99 g Na2PO4-H2O (1M), qs to 1 liter
Mix 342 ml of Na2PO4-2H2O (1M) with 158 ml of Na2PO4-H2O (1M), qs to 500 ml. Adjust pH to 7.2.
Preparation of 1 liter Church’s Buffer – 500 ml phosphate buffer pH 7.2
– 2 ml (0.5 M) EDTA (1mM final)
– 10 g BSA (fraction V) (0.1% final)
– 70 g SDS (7 % final)
Dissolve BSA by adding slowly while mixing to phosphate buffer. Dissolve SDS separately and add to BSA phosphate solution. Add SDS and EDTA – qs to 1 liter.
Sterilize by filtration
Calcium- and magnesium-free Dulbeccos phosphate-buffered saline (CMF-DPBS)
– 8.00 g NaCl (0.137 M)
– 0.20 g KCl (2.7 mM)
– 2.16 g Na2HPO4⋅7H2O (8.1 mM)
– 0.20 g KH2PO4 (1.1 mM)
– 0.10 g MgCl2⋅6H2O (0.5 mM)
– 0.10 g anhydrous CaCl2 (0.9 mM)
– H2O to 1 liter
– Store at room temperature
DTT (dithiothreitol), 1 M
– Dissolve 1.55 g DTT in 10 ml water and filter sterilize. Store in aliquots at −20°C
EDTA (ethylenediaminetetraacetic acid), 0.5 M (pH 8.0)
– Dissolve 186.1 g disodium EDTA dihydrate in 700 ml water.
– Adjust pH to 8.0 with 10 M NaOH (∼50 ml; add slowly).
– Add water to 1 liter and filter sterilize.
– Begin titrating before the sample is completely dissolved. EDTA, even in the disodium salt form, is difficult to dissolve at this concentration unless the pH is increased to between 7 and 8.
Eisen Buffer (10x PBS) (10 Liters)
– 80.0 g NaCl (876.6 gm)
– 2.4 g NaH2PO4 (25.62 gm)
– 14.4 g Na2HPO4 (225 gm)
– H2O to 10 liters
– Dilute 1:10 for use
HBSS (Hanks balanced salt solution)
– 0.40 g KCl (5.4 mM final)
– 0.09 g Na2HPO4⋅7H2O (0.3 mM final)
– 0.06 g KH2PO4 (0.4 mM final)
– 0.35 g NaHCO3 (4.2 mM final)
– 0.14 g CaCl2 (1.3 mM final)
– 0.10 g MgCl2⋅6H2O (0.5 mM final)
– 0.10 g MgSO4⋅7H2O (0.6 mM final)
– 8.0 g NaCl (137 mM final)
– 1.0 g D-glucose (5.6 mM final)
– 0.2 g phenol red (0.02%; optional)
– Add H2O to l liter and adjust pH to 7.4 with 1 M HCl or 1 M NaOH
– Filter sterilize and store up to 1 month at 4°C
HBSS may be made without Ca2+ and Mg2+ (CMF-HBSS).
Bottles should be kept tightly closed to prevent CO2 loss.
HEPES-buffered saline solution, 2× solution
– 16.4 g NaCl
– 11.9 g HEPES acid
– 0.21 g Na2HPO4
– 800 ml H2O
– Titrate to pH 7.05 with 5 M NaOH Add H2O to 1 liter
Filter sterilize through a 0.45-μm nitrocellulose filter Store at −20°C
If the solution is to be used for transfection, the pH should be between 7.05 and 7.12, and should be tested for transfection efficiency.
Lysogeny broth (LB) Medium
Per liter
– 10 g tryptone
– 5 g yeast extract
– 5 g NaCl
– 1 ml 1 M NaOH Autoclave 25 min
Although the pH is adjusted to near 7 with NaOH, the medium is not very highly buffered, and the pH of a culture growing in the medium drops as the culture nears saturation.
The medium may also contain antibiotics (e.g., 50 μg/ml ampicillin, 12 μg/ml tetracycline), galactosides (e.g., 20 μg/ml Xgal, 0.1 mM IPTG), or other nutritional supplements added after the medium has been autoclaved.
To make LB agar for LB plates, add 5 g/liter agar or agarose.
Phosphate buffer, pH 7.2
Preparation of 0.5 M phosphate buffer, pH 7.2
– 177.9 g Na2PO4-2H2O (1M), qs to 1 liter
– 137.99 g Na2PO4-H2O (1M), qs to 1 liter
Mix 342 ml of Na2PO4-2H2O (1M) with 158 ml of Na2PO4-H2O (1M), qs to 500 ml. Adjust pH to 7.2.
Sterilize by filtration
Phosphate-buffered saline (PBS) (1x)
– 8.00 g NaCl (0.137 M)
– 0.20 g KCl (2.7 mM)
– 0.24 g KH2PO4 (1.4 mM)
– 1.44 g Na2HPO4 (0.01 M)
– H2O to 1 liter
Phosphate-buffered saline (PBS) (10x)
– 80.0 g NaCl (137 mM)
– 2.0 g KCl (27 mM)
– 2.4 g KH2PO4 (2.0 mM)
– 14.4 g Na2HPO4 (0.1 M)
– H2O to 1 liter
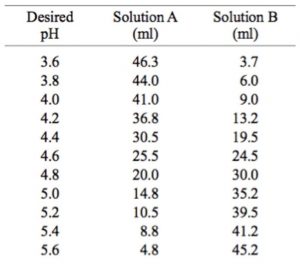
Potassium acetate buffer, 0.1 M
– Solution A: 11.55 ml glacial acetic acid per liter (0.2 M) in water.
– Solution B: 19.6 g potassium acetate (KC2H3O2) per liter (0.2 M) in water.
Preparation of 0.1 M Sodium and Potassium Acetate Buffers
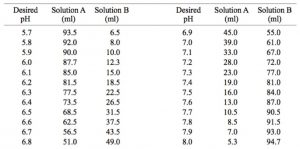
Potassium phosphate buffer, 0.1 M
– Solution A: 27.2 g KH2PO4 per liter (0.2 M final) in water.
– Solution B: 34.8 g K2HPO4 per liter (0.2 M final) in water.
Preparation of 0.1 M Potassium Phosphate Buffers
SDS electrophoresis buffer, 5× solution
– 15.1 g Tris base
– 72.0 g glycine
– 5.0 g SDS
Bring to 1 liter with distilled, deionized H2O. Store up to 1 month at 0° to 4°C. Dilute to 1× before use.
Do not adjust the pH of the stock solution; the pH is 8.3 when diluted to 1×.
SDS sample buffer
Preparation of SDS Sample Buffer
| 2x | 4x | Final conc. in 1x sol. | |
| 0.5 M Tris⋅Cl, pH 6.8 | 2.5 ml | 5 ml | 62.5 mM |
| SDS | 0.4 g | 0.8 g | 2% w/v |
| Glyercol | 2 ml | 4 ml | 10% v/v |
| Bromophenol Blue | 20 mg | 40 mg | 0.1% w/v |
| 2-mercaptoethanol | 400 µl | 800 µl | 300 mM |
| H20 | qs to 10 ml | qs to 10 ml | N/A |
TAE (Tris/acetate/EDTA) electrophoresis buffer, 10× solution
– 224.2 g Tris base
– 5.71 ml glacial acetic acid
– 3.72 g Na2EDTA⋅2H2O
Bring to 1 liter with H2O.
TBE (Tris/borate/EDTA) electrophoresis buffer, 10× solution
– 108 g Tris base
– 55 g boric acid
– 40 ml 0.5 M EDTA, pH 8.0
Bring to 1 liter with H2O.
TBS (Tris-buffered saline)
– 100 mM Tris-HCl, pH 7.5
– 0.9% (w/v) NaCl
Store up to several months at 4°C.
TE (Tris/EDTA) buffer
– 10 mM Tris-HCl, pH 7.4, 7.5, or 8.0
– 1 mM EDTA, pH 8.0
TEA (triethanolamine) solution
– 50 mM triethanolamine, pH ∼11.5
– 0.1% (v/v) Triton X-100
– 0.15 M NaCl
Add Triton X-100 from a 10% stock
