Catalogue
Rabbit anti Human DNA Topoisomerase 1
Catalog number: X2732P$319.00
Add To Cart| Isotype | Ig |
| Product Type |
Antigen Immunoaffinity Purified Polyclonal |
| Units | 100 µg |
| Host | Rabbit |
| Species Reactivity |
Human |
| Application |
Immunohistochemistry (paraffin) |
Background
DNA Topoisomerase 1 releases the supercoiling and torsional tension of DNA introduced during DNA replication and transcription by transiently cleaving and rejoining one strand of the DNA duplex. It introduces a single-strand break via transesterification at a target site in duplex DNA. The scissile phosphodiester is attacked by the catalytic tyrosine of the enzyme, resulting in the formation of a DNA-(3'-phosphotyrosyl)-enzyme intermediate and the expulsion of a 5'-OH DNA strand. The free DNA strand than undergoes passage around the unbroken strand thus removing DNA supercoils. Finally, in the religation step, the DNA 5'-OH attacks the covalent intermediate to expel the active-site tyrosine and restore the DNA phosphodiester backbone By similarity. Regulates the alternative splicing of tissue factor (F3) pre-mRNA in endothelial cells.
Source
Immunogen: Synthetic peptide made to human DNA Topoisomerase 1 [(c)-EVQATDREENKQIALGTSKLN] recognizing a specific sequence within amino acids 692-766.
Product
Product Form: Affinity Purified
Formulation: Provided as solution in phosphate buffered saline with 0.08% sodium azide
Purification Method: Antigen Immunoaffinity Purification
Concentration: See vial for concentration
Applications
Antibody can be used for immunohistochemistry at 5-10 µg/ml on paraffin-embedded tissues. Optimal concentration should be evaluated by serial dilutions.
Positive Control: Found in Endothelial cells.
Storage
Product should be stored at -20°C. Aliquot to avoid freeze/thaw cycles
Product Stability: See expiration date on vial
Shipping Conditions: Ship at ambient temperature, freeze upon arrival
Caution
This product is intended FOR RESEARCH USE ONLY, and FOR TESTS IN VITRO, not for use in diagnostic or therapeutic procedures involving humans or animals. It may contain hazardous ingredients. Please refer to the Safety Data Sheets (SDS) for additional information and proper handling procedures. Dispose product remainders according to local regulations.This datasheet is as accurate as reasonably achievable, but our company accepts no liability for any inaccuracies or omissions in this information.
References
1. Oddou, P., et al. 'Monoclonal antibodies neutralizing mammalian DNA topoisomerase I activity.' Eur. J. Biochem. 1988, 177, 523-529
2. Redinbo, M.R., et al. 'Novel insights into catalytic mechanism from a crystal structure of human topoisomerase I in complex with DNA.' Biochemistry 2000, 39, 6832-6840
3. Eisenreich, A., et al. 'Cdc2-like kinases and DNA topoisomerase I regulate alternative splicing of tissue factor in human endothelial cells.' Circ. Res. 2009, 104, 589-599
Protein Reference(s)
Database Name: UniProt
Accession Number: P11387
Species Accession: Human
Safety Datasheet(s) for this product:
| EA_Sodium Azide |
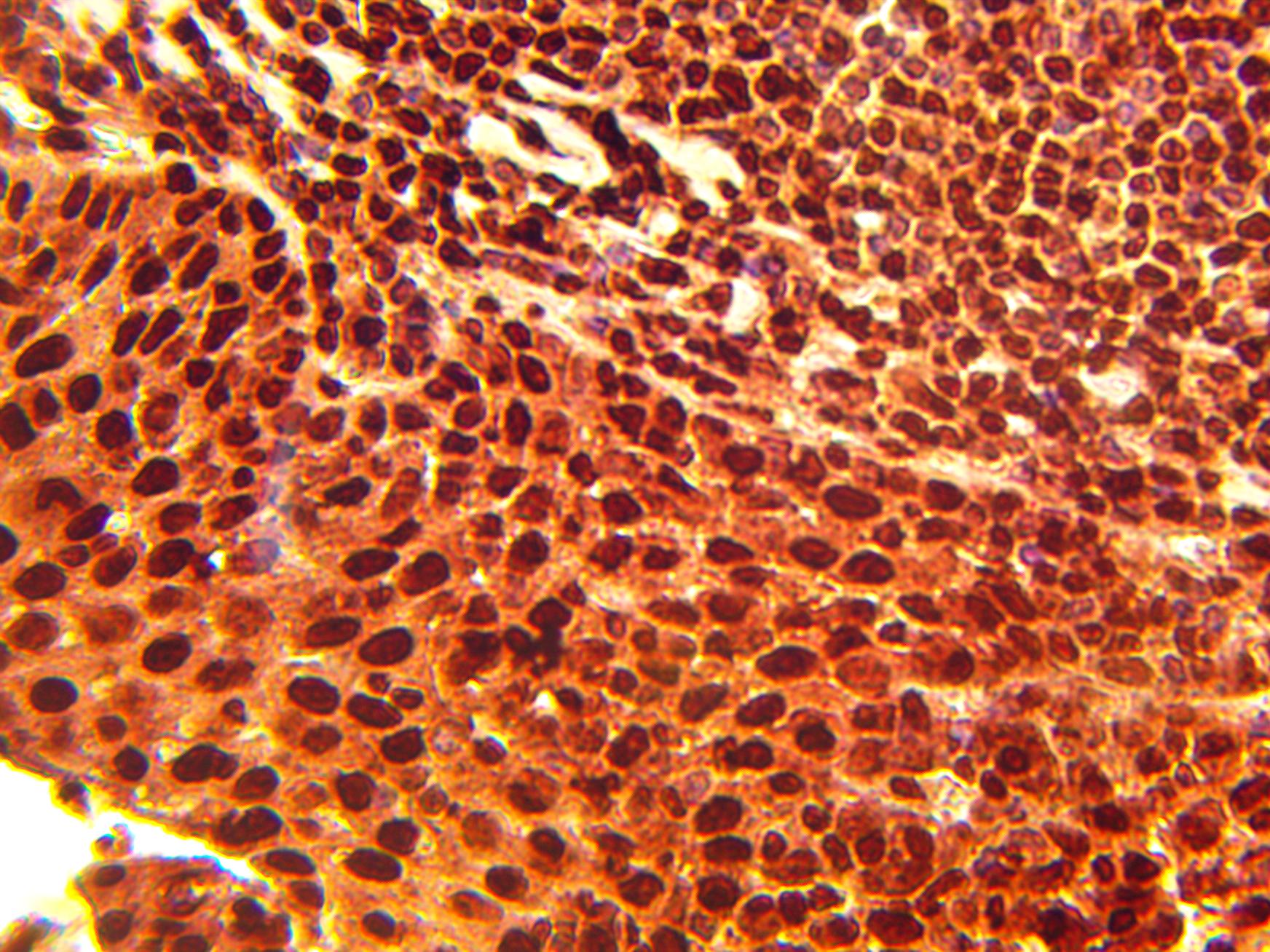
Immunohistochemical staining of normal human tonsil tissue using TOP1 antibody (Cat. No. X2732P) at 10 µg/ml and detected using anti-Rabbit HRP secondary antibody and visualized using DAB substrate and hematoxylin counterstain.
