Catalogue
Mouse anti Human Matrix Metalloproteinase 9 (MMP9)
Catalog number: X2056M$304.00
Add To Cart| Clone | 2C3 |
| Isotype | IgG1 |
| Product Type |
Monoclonal Antibody |
| Units | 200 µg |
| Host | Mouse |
| Species Reactivity |
Human |
| Application |
Immunohistochemistry (paraffin) Western Blotting |
Background
The matrix metalloproteinases (MMP) are a family of peptidase enzymes responsible for the degradation of extracellular matrix components, including collagen, gelatin, fibronectin, laminin and proteoglycan. Transcription of MMP genes is differentially activated by phorbol ester, lipopolysaccharide (LPS) or staphylococcal enterotoxin B (SEB). MMP catalysis requires both calcium and zinc. MMP-9 (also designated 92-kDa type IV collagenase or gelatinase B) has been shown to degrade bone collagens in concert with MMP-1 (also designated interstitial collagenase, fibroblast collagenase or collagenase-1), and cysteine proteases and may play a role in bone osteoclastic resorption. MMP-1 is down-regulated by p53, and abnormality of p53 expression may contribute to joint degradation in rheumatoid arthritis by regulating MMP-1 expression.
Synonyms: CLG4B; GELB; 92 kDa gelatinase; matrix metallopeptidase 9
Source
Hybridoma produced by the fusion of splenocytes from BALB/c mice immunized with a synthetic peptide derived from the C-terminus of the human MMP9 protein and mouse myeloma Ag8563 cells.
Product
Provided as solution in phosphate buffered saline with 0.08% sodium azide
Product Form: Unconjugated
Purification Method: Protein A/G Chromatography
Concentration: See vial for concentration
Applications
Antibody can be used for Western blotting (1-2 µg/ml) and immunohistochemistry on formalin-fixed paraffin-embedded tissues (1-5 µg/ml). Optimal concentration should be evaluated by serial dilutions.
Functional Analysis: Western Blotting
Positive Control: Colon, oesophageal and gastric tissues. Stronger expression in tumor versus normal tissue.
Storage
Product should be stored at -20°C. Aliquot to avoid freeze/thaw cycles
Product Stability: See expiration date on vial
Shipping Conditions: Ship at ambient temperature, freeze upon arrival
Caution
This product is intended FOR RESEARCH USE ONLY, and FOR TESTS IN VITRO, not for use in diagnostic or therapeutic procedures involving humans or animals. It may contain hazardous ingredients. Please refer to the Safety Data Sheets (SDS) for additional information and proper handling procedures. Dispose product remainders according to local regulations.This datasheet is as accurate as reasonably achievable, but our company accepts no liability for any inaccuracies or omissions in this information.
References
1. Wang, L. et al. (2006). Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J. Neurosci. 26(22);5996-6003
2. Solmiari, S.B., et al. (2006). Circulating MMP2 and MMP9 in breast cancer -- potential role in classification of patients into low risk, high risk, benign disease and breast cancer categories. Int. J. Cancer. 119(6);1403-1411
3. Chen, X., et al. (2005). Increased plasma MMP9 in integrin alpha1-null mice enhances lung metastasis of colon carcinoma cells. Int. J. Cancer. 116(1);52-61
Protein Reference(s)
Database Name: UniProt
Accession Number: P14780 (Human)
Species Accession: Human
Safety Datasheet(s) for this product:
| EA_Sodium Azide |
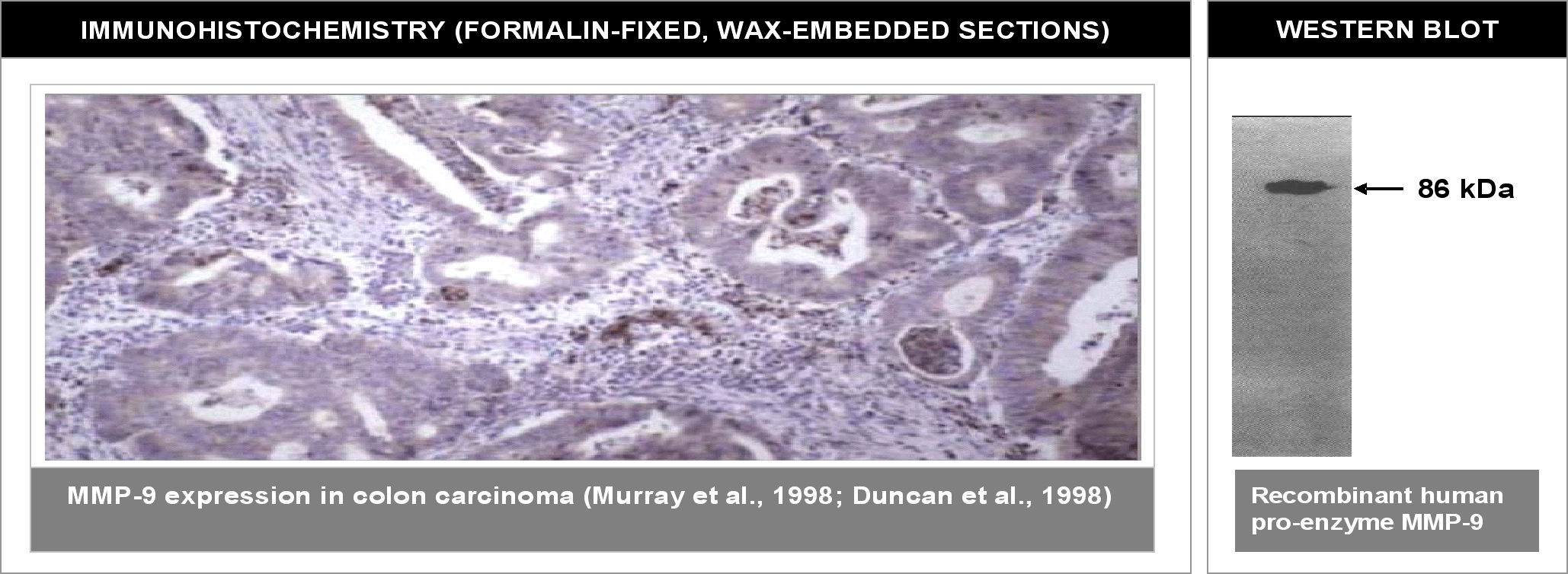
" Left: Immunohistochemical staining of paraffin embedded colon carcinoma using MMP9 antibody (Cat. No. X2056M). Antigen retreival using 10mM citrate buffer and microwave method was used. Right: Western blot using MMP9 antibody on recombinant human proenzyme MMP9 (400 ng/lane). "
