Catalogue
Mouse anti Human CD15-NA FITC
Catalog number: X1496M$325.00
Add To Cart| Clone | MCS-1 |
| Isotype | IgG3 |
| Product Type |
Single-Color Reagent |
| Units | 50 Tests |
| Host | Mouse |
| Species Reactivity |
Human |
| Application |
Flow Cytometry |
Background
CD15-FITC is a monoclonal antibody (MAb) labelled with fluorescein isothiocyanate (FITC) designed for use as a direct immunofluorescence reagent in the identification and enumeration of cells which express the CD15 antigen by flow cytometry. The CD15 MAb is applied for the initial evaluation of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) together with a panel of other antibodies. Interpretation of results must be made within the context of the patientÕs clinical history and other diagnostic tests by a certified professional.
Synonyms: Non-Agglutinating CD15, FITC Labeled
Product
Provided as an aqueous buffered solution containing protein stabilizer and 0.09% sodium azide.
Product Form: FITC
Purification Method: Affinity Chromatography
Concentration: Titered for flow cytometry
Applications
This CD15 antibody is an IgG3 antibody as compared to other CD15 antibodies available which are IgM’s. These IgM’s cause the cells labeled with them to aggregate in the cytometer and read as one cell, producing lower numbers of cells to be reported. Our CD15 antibody does not cause the cells to aggregate, yielding much more accurate results.
Functional Analysis: Flow Cytometry Staining
Storage
Product should be stored at 4-8°C. DO NOT FREEZE
Product Stability: See expiration date on vial
Shipping Conditions: Ship at ambient temperature, do not freeze, refrigerate upon arrival
Caution
This product is intended FOR RESEARCH USE ONLY, and FOR TESTS IN VITRO, not for use in diagnostic or therapeutic procedures involving humans or animals. It may contain hazardous ingredients. Please refer to the Safety Data Sheets (SDS) for additional information and proper handling procedures. Dispose product remainders according to local regulations.This datasheet is as accurate as reasonably achievable, but our company accepts no liability for any inaccuracies or omissions in this information.
References
1. Ball ED. M5. CD15 cluster workshop report. In: Schlossman SF, Boumsell L, Gilks W, Harlan JM, Kishimoto T, Morimoto C, Ritz J, et al. editors. Leucocyte typing V. White cell differentiation antigens. Proceedings of Fifth International Workshop and conference held in Boston, USA 3-7 november, 1993. Volume One. Oxford University Press (1995).
2. Bahia DMM, Yamamoto M, Chauffaille MLF, Kimura EYS, Bordin JO, et al. Aberrant phenotypes in acute myeloid leukemia: a high frequency and clinical significance. Haematologica.86: 801-806 (2001)
3. van Gongen JJM, Adriaansen HJ. Immunobiology of leukemia. In Henderson ES, Lister TA, Greaves MF editors. Leukemia. WB Saunders Company (1996)
4. Menéndez P, et al. Comparison between a lyse-and-then-wash method and a lyse-non-wash technique for the enumeration of CD34+ hematopoietic progenitor cells. Cytometry (Comm. Clin. Cytometry) 34: 264-271 (1998)
5. GratamaJW, Menéndez P, Kraan J, Orfao A. Loss of CD34+ hematopoietic progenitor cells due to washing can be reduced by the use of fixative-free erytrocyte lysing reagents. J Immunol. Methods 239: 13-23 (2000)
6. Protection of Laboratory Workers from occupationally acquired infections. Second edition; approved guideline (2001). Villanova PA: National Committee for Clinical Laboratory Standards; Document M29-A2.
7. Procedures for the collection of diagnostic blood specimens by venipuncture- approved standard; Fifth edition (2003). Wayne PA: National Committee for Clinical Laboratory Standards; Document H3-A5.
8. Clinical applications of flow cytometry: Quality assurance and immunophenotyping of lymphocytes; approved guideline (1998). Wayne PA: National Committee for Clinical Laboratory Standards; Document H42-A.
9. Braylan RC, Orfao A, Borowitz MJ, Davis BH. Optimal number of reagents required to evaluate hematolymphoid neoplasias: results of an international consensus meeting. Cytometry 46: 23-7 (2001)
10. Jennings CD, Foon KA. Recent advances in flow cytometry: application to the diagnosis of hematologic malignancy. Blood 90(8): 2863-2892 (1997)
11. Orfao A, Ortuño F, de Santiago M, López A, San Miguel J. Immunophenotyping of acute leucemias and myelodysplastic syndromes. Cytometry Part A 58A: 62-71 (2004)
12. Reichert et al. Lymphocyte subset reference ranges in adult Caucasians. Clin Immunol Immunopathol 60:190-208 (1991)
Safety Datasheet(s) for this product:
| EA_Sodium Azide |
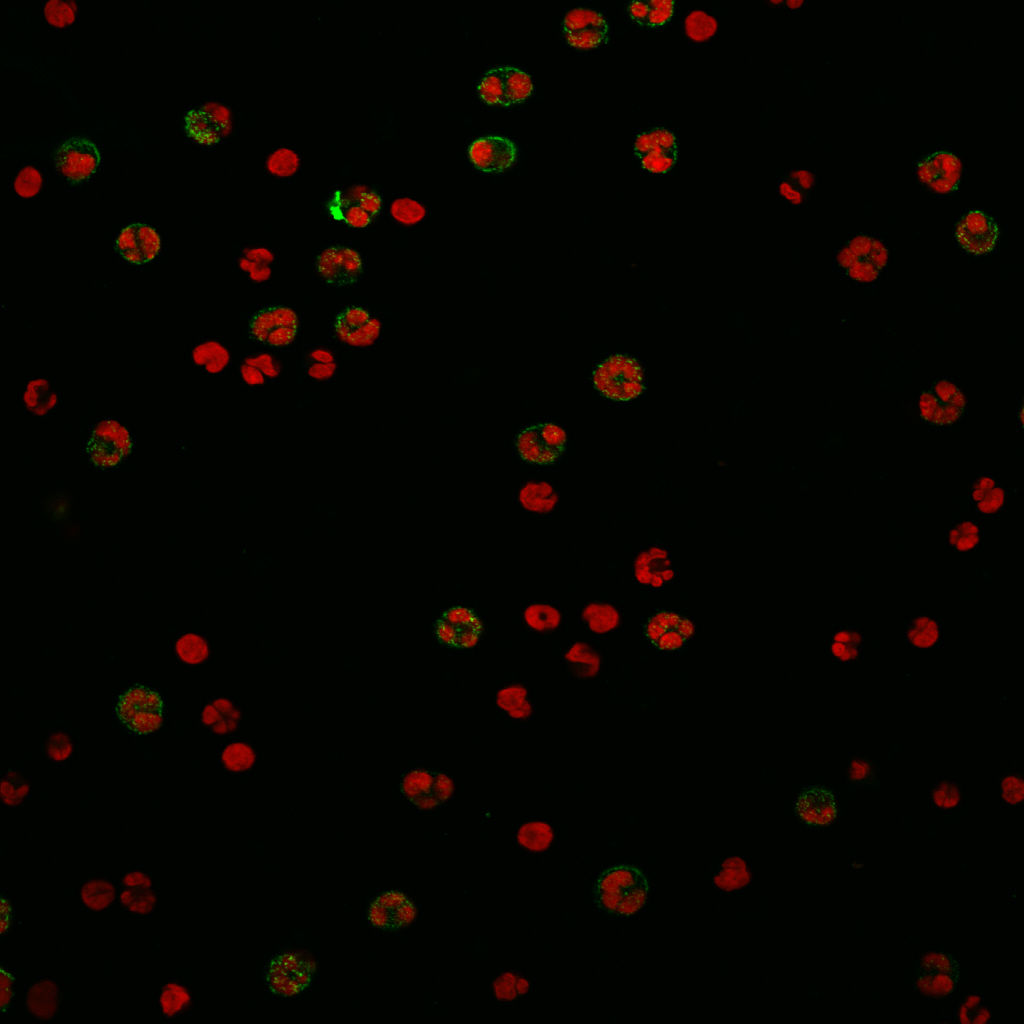
Immunofluorescence staining using CD15-FITC IgG3 antibody (Cat. No. X1496M) on human neutrophils.
